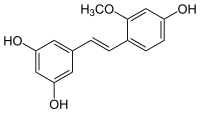
Gnetucleistol D
 | |
| Names | |
|---|---|
| Preferred IUPAC name 5-[(E)-2-(4-Hydroxy-2-methoxyphenyl)ethen-1-yl]benzene-1,3-diol | |
| Other names 2-methoxyoxyresveratrol | |
| Identifiers | |
CAS Number |
|
3D model (JSmol) |
|
| ChEMBL |
|
| ChemSpider |
|
PubChem CID |
|
| UNII |
|
CompTox Dashboard (EPA) |
|
InChI
| |
| |
| Properties | |
Chemical formula | C15H14O4 |
| Molar mass | 258.27 g/mol |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). Infobox references | |
Chemical compound
Gnetucleistol D is a stilbenoid found in the Chinese herb Gnetum cleistostachyum.[1]
References
- ^ Yao Chun-Suo; Lin Mao; Liu Xin; Wang Ying-Hong (15 August 2003). "Stilbenes from Gnetum cleistostachyum". Acta Chimica Sinica. 61 (8): 1331–1334.
- v
- t
- e
Hydroxystilbenes and their glycosides (monomeric forms)
- Pinosylvin
- 3,4′-Dihydroxystilbene
- Resveratrol
| |
| Combretastatins | |
|---|---|
| |||||
| |||||
 | This article about an aromatic compound is a stub. You can help Wikipedia by expanding it. |
- v
- t
- e













