Liquiritin
 | |
| Names | |
|---|---|
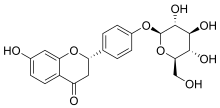
| IUPAC name (2S)-4′-(β-D-Glucopyranosyloxy)-7-hydroxyflavan-4-one | |
| Systematic IUPAC name (2S)-7-Hydroxy-4-(4-{[(2S,3R,4S,5S,6R)-3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl]oxy}phenyl)-2,3-dihydro-4H-1-benzopyran-4-one | |
| Other names Liquiritoside Liquiritigenin-4'-O-glucoside | |
| Identifiers | |
CAS Number |
|
3D model (JSmol) |
|
| ChEBI |
|
| ChemSpider |
|
PubChem CID |
|
| UNII |
|
CompTox Dashboard (EPA) |
|
InChI
| |
| |
| Properties | |
Chemical formula | C21H22O9 |
| Molar mass | 418.398 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). Infobox references | |
Chemical compound
Liquiritin is the 4'-O-glucoside of the flavanone liquiritigenin. Liquiritin is one of flavone compounds derived from licorice.[1]
References
- ^ Cong JX, Wang SY, Wu XH, Yu P (2012). "Optimization of Separation Conditions of Liquiritin in Preparative Liquid Chromatography". Advanced Materials Research. 550–553: 1647–1652. doi:10.4028/www.scientific.net/AMR.550-553.1647. S2CID 97214921.
Further reading
- Wang J, Wang D, Yu J, Liu C, Li L, Zhang Y (April 2014). "Isolation of liquiritigenin-4'-apiosyl-glucoside and liquiritin from the root of Glycyrrhiza uralensis by high-performance centrifugal partition chromatography". Journal of Chromatographic Science. 52 (4): 310–4. doi:10.1093/chromsci/bmt029. PMID 23552847.
- Cong, Jing Xiang; Wang, Shao Yan; Gao, Hong (2012). "Separation of Liquiritin by Two-Dimensional Liquid Chromatography". Advanced Materials Research. 455–456: 1232–1238. doi:10.4028/www.scientific.net/AMR.455-456.1232. ISSN 1662-8985. S2CID 135570958.
- Cong J, Lin B (March 2007). "Separation of Liquiritin by simulated moving bed chromatography". Journal of Chromatography A. 1145 (1–2): 190–4. doi:10.1016/j.chroma.2007.01.088. PMID 17289063.
- Ni H, Xu M, Xie K, Fei Y, Deng H, He Q, Wang T, Liu S, Zhu J, Xu L, Yao M (2020). "Liquiritin Alleviates Pain Through Inhibiting CXCL1/CXCR2 Signaling Pathway in Bone Cancer Pain Rat". Frontiers in Pharmacology. 11: 436. doi:10.3389/fphar.2020.00436. PMC 7193085. PMID 32390832.
External links
 Media related to Liquiritin at Wikimedia Commons
Media related to Liquiritin at Wikimedia Commons
- v
- t
- e
Flavanones and their glycosides
- Butin
- Eriodictyol
- Naringenin
- Pinocembrin
- Alpinetin
- Hesperetin
- Homoeriodictyol
- Isosakuranetin
- Pinostrobin (Pinocembrin-7-methylether)
- Sakuranetin
- Sterubin
- Eriocitrin
- Neoeriocitrin
- Hesperidin
- Liquiritin
- Naringin (Narigenin 7-O-neohesperidoside)
- Narirutin (Naringenin 7-O-rutinoside)
- Poncirin
- Prunin (Naringenin-7-O-glucoside)
- Sakuranin
- Nirurin
 | This article about an aromatic compound is a stub. You can help Wikipedia by expanding it. |
- v
- t
- e












