Protein-coding gene in the species Homo sapiens
| MSN |
|---|
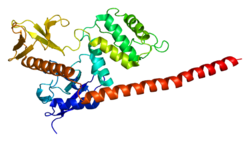 |
| Available structures |
|---|
| PDB | Ortholog search: PDBe RCSB |
|---|
| List of PDB id codes |
|---|
1E5W, 1EF1, 1SGH |
|
|
| Identifiers |
|---|
| Aliases | MSN, HEL70, moesin, IMD50 |
|---|
| External IDs | OMIM: 309845; MGI: 97167; HomoloGene: 1833; GeneCards: MSN; OMA:MSN - orthologs |
|---|
| Gene location (Human) |
|---|
 | | Chr. | X chromosome (human)[1] |
|---|
| | Band | Xq12 | Start | 65,588,377 bp[1] |
|---|
| End | 65,741,931 bp[1] |
|---|
|
| Gene location (Mouse) |
|---|
 | | Chr. | X chromosome (mouse)[2] |
|---|
| | Band | X|X C3 | Start | 95,139,648 bp[2] |
|---|
| End | 95,212,158 bp[2] |
|---|
|
| RNA expression pattern |
|---|
| Bgee | | Human | Mouse (ortholog) |
|---|
| Top expressed in | - lower lobe of lung
- white blood cell
- monocyte
- granulocyte
- synovial joint
- saphenous vein
- synovial membrane
- right lung
- appendix
- smooth muscle tissue
|
| | Top expressed in | - right lung
- right lung lobe
- left lung
- endothelial cell of lymphatic vessel
- left lung lobe
- granulocyte
- mesenteric lymph nodes
- stroma of bone marrow
- ascending aorta
- tibiofemoral joint
|
| | More reference expression data |
|
|---|
| BioGPS |  | | More reference expression data |
|
|---|
|
| Gene ontology |
|---|
| Molecular function | - cytoskeletal protein binding
- structural constituent of cytoskeleton
- protein binding
- actin binding
- double-stranded RNA binding
- signaling receptor binding
- protein kinase binding
- cell adhesion molecule binding
- enzyme binding
| | Cellular component | - cytoplasm
- vesicle
- cell projection
- pseudopodium
- blood microparticle
- membrane
- focal adhesion
- filopodium
- myelin sheath
- plasma membrane
- apical part of cell
- microvillus
- uropod
- basolateral plasma membrane
- apical plasma membrane
- perinuclear region of cytoplasm
- extracellular exosome
- cytoskeleton
- microvillus membrane
- nucleus
- cell periphery
- extracellular space
- cell surface
- cytosol
| | Biological process | - leukocyte cell-cell adhesion
- regulation of cell size
- establishment of endothelial barrier
- regulation of organelle assembly
- cellular response to testosterone stimulus
- regulation of lymphocyte migration
- positive regulation of podosome assembly
- gland morphogenesis
- positive regulation of gene expression
- establishment of epithelial cell apical/basal polarity
- regulation of cell shape
- membrane to membrane docking
- positive regulation of protein localization to early endosome
- positive regulation of early endosome to late endosome transport
- leukocyte migration
- cytoskeleton organization
- immunological synapse formation
- T cell proliferation
- T cell aggregation
- T cell migration
- interleukin-12-mediated signaling pathway
- viral process
| | Sources:Amigo / QuickGO |
|
|
| Wikidata |
| View/Edit Human | View/Edit Mouse |
|
Moesin is a protein that in humans is encoded by the MSN gene.[5][6]
Moesin (for membrane-organizing extension spike protein) is a member of the ERM protein family which includes ezrin and radixin. ERM proteins appear to function as cross-linkers between plasma membranes and actin-based cytoskeletons.[7]
Moesin is localized to filopodia and other membranous protrusions that are important for cell–cell recognition and signaling and for cell movement.[7]
Moesin has FERM domain at N-terminal.
Interactions
Moesin has been shown to interact with:
References
- ^ a b c GRCh38: Ensembl release 89: ENSG00000147065 – Ensembl, May 2017
- ^ a b c GRCm38: Ensembl release 89: ENSMUSG00000031207 – Ensembl, May 2017
- ^ "Human PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- ^ "Mouse PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- ^ Lankes WT, Furthmayr H (Oct 1991). "Moesin: a member of the protein 4.1-talin-ezrin family of proteins". Proc. Natl. Acad. Sci. U.S.A. 88 (19): 8297–301. Bibcode:1991PNAS...88.8297L. doi:10.1073/pnas.88.19.8297. PMC 52495. PMID 1924289.
- ^ Amieva MR, Furthmayr H (Sep 1995). "Subcellular localization of moesin in dynamic filopodia, retraction fibers, and other structures involved in substrate exploration, attachment, and cell-cell contacts". Exp. Cell Res. 219 (1): 180–96. doi:10.1006/excr.1995.1218. PMID 7628534.
- ^ a b "Entrez Gene: MSN moesin".
- ^ Serrador JM, Nieto M, Alonso-Lebrero JL, del Pozo MA, Calvo J, Furthmayr H, Schwartz-Albiez R, Lozano F, González-Amaro R, Sánchez-Mateos P, Sánchez-Madrid F (Jun 1998). "CD43 interacts with moesin and ezrin and regulates its redistribution to the uropods of T lymphocytes at the cell-cell contacts". Blood. 91 (12): 4632–44. doi:10.1182/blood.V91.12.4632. PMID 9616160.
- ^ Yonemura S, Hirao M, Doi Y, Takahashi N, Kondo T, Tsukita S, Tsukita S (Feb 1998). "Ezrin/radixin/moesin (ERM) proteins bind to a positively charged amino acid cluster in the juxta-membrane cytoplasmic domain of CD44, CD43, and ICAM-2". J. Cell Biol. 140 (4): 885–95. doi:10.1083/jcb.140.4.885. PMC 2141743. PMID 9472040.
- ^ Serrador JM, Alonso-Lebrero JL, del Pozo MA, Furthmayr H, Schwartz-Albiez R, Calvo J, Lozano F, Sánchez-Madrid F (Sep 1997). "Moesin interacts with the cytoplasmic region of intercellular adhesion molecule-3 and is redistributed to the uropod of T lymphocytes during cell polarization". J. Cell Biol. 138 (6): 1409–23. doi:10.1083/jcb.138.6.1409. PMC 2132557. PMID 9298994.
- ^ Serrador JM, Vicente-Manzanares M, Calvo J, Barreiro O, Montoya MC, Schwartz-Albiez R, Furthmayr H, Lozano F, Sánchez-Madrid F (Mar 2002). "A novel serine-rich motif in the intercellular adhesion molecule 3 is critical for its ezrin/radixin/moesin-directed subcellular targeting". J. Biol. Chem. 277 (12): 10400–9. doi:10.1074/jbc.M110694200. PMID 11784723.
- ^ a b Wientjes FB, Reeves EP, Soskic V, Furthmayr H, Segal AW (Nov 2001). "The NADPH oxidase components p47(phox) and p40(phox) bind to moesin through their PX domain". Biochem. Biophys. Res. Commun. 289 (2): 382–8. doi:10.1006/bbrc.2001.5982. PMID 11716484.
- ^ Barreiro O, Yanez-Mo M, Serrador JM, Montoya MC, Vicente-Manzanares M, Tejedor R, Furthmayr H, Sanchez-Madrid F (Jun 2002). "Dynamic interaction of VCAM-1 and ICAM-1 with moesin and ezrin in a novel endothelial docking structure for adherent leukocytes". J. Cell Biol. 157 (7): 1233–45. doi:10.1083/jcb.200112126. PMC 2173557. PMID 12082081.
- ^ Gajate C, Mollinedo F (Mar 2005). "Cytoskeleton-mediated death receptor and ligand concentration in lipid rafts forms apoptosis-promoting clusters in cancer chemotherapy". J. Biol. Chem. 280 (12): 11641–7. doi:10.1074/jbc.M411781200. PMID 15659383.
- ^ Gary R, Bretscher A (Aug 1995). "Ezrin self-association involves binding of an N-terminal domain to a normally masked C-terminal domain that includes the F-actin binding site". Mol. Biol. Cell. 6 (8): 1061–75. doi:10.1091/mbc.6.8.1061. PMC 301263. PMID 7579708.
- ^ Gary R, Bretscher A (Nov 1993). "Heterotypic and homotypic associations between ezrin and moesin, two putative membrane-cytoskeletal linking proteins". Proc. Natl. Acad. Sci. U.S.A. 90 (22): 10846–50. Bibcode:1993PNAS...9010846G. doi:10.1073/pnas.90.22.10846. PMC 47875. PMID 8248180.
Further reading
- Tsukita S, Yonemura S (1997). "ERM (ezrin/radixin/moesin) family: from cytoskeleton to signal transduction". Curr. Opin. Cell Biol. 9 (1): 70–5. doi:10.1016/S0955-0674(97)80154-8. PMID 9013673.
- Vaheri A, Carpén O, Heiska L, Helander TS, Jääskeläinen J, Majander-Nordenswan P, Sainio M, Timonen T, Turunen O (1997). "The ezrin protein family: membrane-cytoskeleton interactions and disease associations". Curr. Opin. Cell Biol. 9 (5): 659–66. doi:10.1016/S0955-0674(97)80119-6. PMID 9330869.
- Matarrese P, Malorni W (2005). "Human immunodeficiency virus (HIV)-1 proteins and cytoskeleton: partners in viral life and host cell death". Cell Death Differ. 12 (Suppl 1): 932–41. doi:10.1038/sj.cdd.4401582. PMID 15818415.
- Gary R, Bretscher A (1995). "Ezrin self-association involves binding of an N-terminal domain to a normally masked C-terminal domain that includes the F-actin binding site". Mol. Biol. Cell. 6 (8): 1061–75. doi:10.1091/mbc.6.8.1061. PMC 301263. PMID 7579708.
- Schwartz-Albiez R, Merling A, Spring H, Möller P, Koretz K (1995). "Differential expression of the microspike-associated protein moesin in human tissues". Eur. J. Cell Biol. 67 (3): 189–98. PMID 7588875.
- Schneider-Schaulies J, Dunster LM, Schwartz-Albiez R, Krohne G, ter Meulen V (1995). "Physical association of moesin and CD46 as a receptor complex for measles virus". J. Virol. 69 (4): 2248–56. doi:10.1128/JVI.69.4.2248-2256.1995. PMC 188894. PMID 7884872.
- Wilgenbus KK, Hsieh CL, Lankes WT, Milatovich A, Francke U, Furthmayr H (1994). "Structure and localization on the X chromosome of the gene coding for the human filopodial protein moesin (MSN)". Genomics. 19 (2): 326–33. doi:10.1006/geno.1994.1065. PMID 8188263.
- Gary R, Bretscher A (1993). "Heterotypic and homotypic associations between ezrin and moesin, two putative membrane-cytoskeletal linking proteins". Proc. Natl. Acad. Sci. U.S.A. 90 (22): 10846–50. Bibcode:1993PNAS...9010846G. doi:10.1073/pnas.90.22.10846. PMC 47875. PMID 8248180.
- Dunster LM, Schneider-Schaulies J, Löffler S, Lankes W, Schwartz-Albiez R, Lottspeich F, ter Meulen V (1994). "Moesin: a cell membrane protein linked with susceptibility to measles virus infection". Virology. 198 (1): 265–74. doi:10.1006/viro.1994.1029. PMID 8259662.
- Nakamura F, Amieva MR, Furthmayr H (1995). "Phosphorylation of threonine 558 in the carboxyl-terminal actin-binding domain of moesin by thrombin activation of human platelets". J. Biol. Chem. 270 (52): 31377–85. doi:10.1074/jbc.270.52.31377. PMID 8537411.
- Ott DE, Coren LV, Kane BP, Busch LK, Johnson DG, Sowder RC, Chertova EN, Arthur LO, Henderson LE (1996). "Cytoskeletal proteins inside human immunodeficiency virus type 1 virions". J. Virol. 70 (11): 7734–43. doi:10.1128/JVI.70.11.7734-7743.1996. PMC 190843. PMID 8892894.
- Hecker C, Weise C, Schneider-Schaulies J, Holmes HC, ter Meulen V (1997). "Specific binding of HIV-1 envelope protein gp120 to the structural membrane proteins ezrin and moesin". Virus Res. 49 (2): 215–23. doi:10.1016/S0168-1702(97)00039-7. PMC 7126478. PMID 9213396.
- Serrador JM, Alonso-Lebrero JL, del Pozo MA, Furthmayr H, Schwartz-Albiez R, Calvo J, Lozano F, Sánchez-Madrid F (1997). "Moesin interacts with the cytoplasmic region of intercellular adhesion molecule-3 and is redistributed to the uropod of T lymphocytes during cell polarization". J. Cell Biol. 138 (6): 1409–23. doi:10.1083/jcb.138.6.1409. PMC 2132557. PMID 9298994.
- Reczek D, Berryman M, Bretscher A (1997). "Identification of EBP50: A PDZ-containing phosphoprotein that associates with members of the ezrin-radixin-moesin family". J. Cell Biol. 139 (1): 169–79. doi:10.1083/jcb.139.1.169. PMC 2139813. PMID 9314537.
- Murthy A, Gonzalez-Agosti C, Cordero E, Pinney D, Candia C, Solomon F, Gusella J, Ramesh V (1998). "NHE-RF, a regulatory cofactor for Na(+)-H+ exchange, is a common interactor for merlin and ERM (MERM) proteins". J. Biol. Chem. 273 (3): 1273–6. doi:10.1074/jbc.273.3.1273. PMID 9430655.
- Yonemura S, Hirao M, Doi Y, Takahashi N, Kondo T, Tsukita S, Tsukita S (1998). "Ezrin/radixin/moesin (ERM) proteins bind to a positively charged amino acid cluster in the juxta-membrane cytoplasmic domain of CD44, CD43, and ICAM-2". J. Cell Biol. 140 (4): 885–95. doi:10.1083/jcb.140.4.885. PMC 2141743. PMID 9472040.
PDB gallery
-
1e5w: STRUCTURE OF ISOLATED FERM DOMAIN AND FIRST LONG HELIX OF MOESIN -
1ef1: CRYSTAL STRUCTURE OF THE MOESIN FERM DOMAIN/TAIL DOMAIN COMPLEX -
1j19: Crystal structure of the radxin FERM domain complexed with the ICAM-2 cytoplasmic peptide -
1sgh: Moesin FERM domain bound to EBP50 C-terminal peptide -
2d10: Crystal structure of the Radixin FERM domain complexed with the NHERF-1 C-terminal tail peptide -
2d11: Crystal structure of the Radixin FERM domain complexed with the NHERF-2 C-terminal tail peptide -
2d2q: Crystal structure of the dimerized radixin FERM domain -
2yvc: Crystal structure of the Radixin FERM domain complexed with the NEP cytoplasmic tail |
This article incorporates text from the United States National Library of Medicine, which is in the public domain.

 1e5w: STRUCTURE OF ISOLATED FERM DOMAIN AND FIRST LONG HELIX OF MOESIN
1e5w: STRUCTURE OF ISOLATED FERM DOMAIN AND FIRST LONG HELIX OF MOESIN 1ef1: CRYSTAL STRUCTURE OF THE MOESIN FERM DOMAIN/TAIL DOMAIN COMPLEX
1ef1: CRYSTAL STRUCTURE OF THE MOESIN FERM DOMAIN/TAIL DOMAIN COMPLEX 1j19: Crystal structure of the radxin FERM domain complexed with the ICAM-2 cytoplasmic peptide
1j19: Crystal structure of the radxin FERM domain complexed with the ICAM-2 cytoplasmic peptide 1sgh: Moesin FERM domain bound to EBP50 C-terminal peptide
1sgh: Moesin FERM domain bound to EBP50 C-terminal peptide 2d10: Crystal structure of the Radixin FERM domain complexed with the NHERF-1 C-terminal tail peptide
2d10: Crystal structure of the Radixin FERM domain complexed with the NHERF-1 C-terminal tail peptide 2d11: Crystal structure of the Radixin FERM domain complexed with the NHERF-2 C-terminal tail peptide
2d11: Crystal structure of the Radixin FERM domain complexed with the NHERF-2 C-terminal tail peptide 2d2q: Crystal structure of the dimerized radixin FERM domain
2d2q: Crystal structure of the dimerized radixin FERM domain 2yvc: Crystal structure of the Radixin FERM domain complexed with the NEP cytoplasmic tail
2yvc: Crystal structure of the Radixin FERM domain complexed with the NEP cytoplasmic tail

























