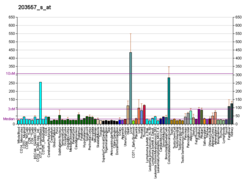
PCBD1
| PCBD1 | |||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 | |||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| Identifiers | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Aliases | PCBD1, DCOH, PCBD, PCD, PHS, pterin-4 alpha-carbinolamine dehydratase 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||
| External IDs | OMIM: 126090; MGI: 94873; HomoloGene: 57028; GeneCards: PCBD1; OMA:PCBD1 - orthologs | ||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| Wikidata | |||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
Pterin-4-alpha-carbinolamine dehydratase is an enzyme that in humans is encoded by the PCBD1 gene.[5][6]
Function
This gene encodes pterin-4 alpha-carbinolamine dehydratase, an enzyme involved in phenylalanine hydroxylation. The enzyme regulates the homodimerization of the transcription factor hepatocyte nuclear factor 1 (HNF1).[6]
Clinical significance
Mutations of the PCBD1 gene cause pterin-4 alpha-carbinolamine dehydratase deficiency, one of the forms of tetrahydrobiopterin deficiency.[7]
Interactions
PCBD1 has been shown to interact with DYRK1B[8] and HNF1A.[9][10]
References
- ^ a b c GRCh38: Ensembl release 89: ENSG00000166228 – Ensembl, May 2017
- ^ a b c GRCm38: Ensembl release 89: ENSMUSG00000020098 – Ensembl, May 2017
- ^ "Human PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- ^ "Mouse PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- ^ Milatovich A, Mendel DB, Crabtree GR, Francke U (April 1993). "Genes for the dimerization cofactor of hepatocyte nuclear factor-1 alpha (DCOH) are on human and murine chromosomes 10". Genomics. 16 (1): 292–295. doi:10.1006/geno.1993.1182. PMID 8486378.
- ^ a b "Entrez Gene: PCBD1 pterin-4 alpha-carbinolamine dehydratase/dimerization cofactor of hepatocyte nuclear factor 1 alpha (TCF1)".
- ^ Opladen T, López-Laso E, Cortès-Saladelafont E, Pearson TS, Sivri HS, Yildiz Y, et al. (May 2020). "Consensus guideline for the diagnosis and treatment of tetrahydrobiopterin (BH4) deficiencies". Orphanet Journal of Rare Diseases. 15 (1): 126. doi:10.1186/s13023-020-01379-8. PMC 7251883. PMID 32456656.
- ^ Lim S, Jin K, Friedman E (July 2002). "Mirk protein kinase is activated by MKK3 and functions as a transcriptional activator of HNF1alpha". The Journal of Biological Chemistry. 277 (28): 25040–25046. doi:10.1074/jbc.M203257200. PMID 11980910.
- ^ Ewing RM, Chu P, Elisma F, Li H, Taylor P, Climie S, et al. (2007). "Large-scale mapping of human protein-protein interactions by mass spectrometry". Molecular Systems Biology. 3 (1): 89. doi:10.1038/msb4100134. PMC 1847948. PMID 17353931.
- ^ Sourdive DJ, Transy C, Garbay S, Yaniv M (April 1997). "The bifunctional DCOH protein binds to HNF1 independently of its 4-alpha-carbinolamine dehydratase activity". Nucleic Acids Research. 25 (8): 1476–1484. doi:10.1093/nar/25.8.1476. PMC 146627. PMID 9092652.
Further reading
- Hansen LP, Crabtree GR (April 1993). "Regulation of the HNF-1 homeodomain proteins by DCoH". Current Opinion in Genetics & Development. 3 (2): 246–253. doi:10.1016/0959-437X(93)90030-S. PMID 8504250.
- Suck D, Ficner R (June 1996). "Structure and function of PCD/DCoH, an enzyme with regulatory properties". FEBS Letters. 389 (1): 35–39. doi:10.1016/0014-5793(96)00573-X. PMID 8682201. S2CID 43378844.
- Thöny B, Auerbach G, Blau N (April 2000). "Tetrahydrobiopterin biosynthesis, regeneration and functions". The Biochemical Journal. 347 Pt 1 (1): 1–16. doi:10.1042/0264-6021:3470001. PMC 1220924. PMID 10727395.
- Mendel DB, Khavari PA, Conley PB, Graves MK, Hansen LP, Admon A, Crabtree GR (December 1991). "Characterization of a cofactor that regulates dimerization of a mammalian homeodomain protein". Science. 254 (5039): 1762–1767. Bibcode:1991Sci...254.1762M. doi:10.1126/science.1763325. PMID 1763325.
- Thöny B, Neuheiser F, Blau N, Heizmann CW (May 1995). "Characterization of the human PCBD gene encoding the bifunctional protein pterin-4 alpha-carbinolamine dehydratase/dimerization cofactor for the transcription factor HNF-1 alpha". Biochemical and Biophysical Research Communications. 210 (3): 966–973. doi:10.1006/bbrc.1995.1751. PMID 7763270.
- Thöny B, Heizmann CW, Mattei MG (January 1994). "Chromosomal location of two human genes encoding tetrahydrobiopterin-metabolizing enzymes: 6-pyruvoyl-tetrahydropterin synthase maps to 11q22.3-q23.3, and pterin-4 alpha-carbinolamine dehydratase maps to 10q22". Genomics. 19 (2): 365–368. doi:10.1006/geno.1994.1071. PMID 8188266.
- Citron BA, Kaufman S, Milstien S, Naylor EW, Greene CL, Davis MD (September 1993). "Mutation in the 4a-carbinolamine dehydratase gene leads to mild hyperphenylalaninemia with defective cofactor metabolism". American Journal of Human Genetics. 53 (3): 768–774. PMC 1682436. PMID 8352282.
- Hauer CR, Rebrin I, Thöny B, Neuheiser F, Curtius HC, Hunziker P, et al. (March 1993). "Phenylalanine hydroxylase-stimulating protein/pterin-4 alpha-carbinolamine dehydratase from rat and human liver. Purification, characterization, and complete amino acid sequence". The Journal of Biological Chemistry. 268 (7): 4828–4831. doi:10.1016/S0021-9258(18)53471-2. PMID 8444860.
- Johnen G, Kowlessur D, Citron BA, Kaufman S (December 1995). "Characterization of the wild-type form of 4a-carbinolamine dehydratase and two naturally occurring mutants associated with hyperphenylalaninemia". Proceedings of the National Academy of Sciences of the United States of America. 92 (26): 12384–12388. Bibcode:1995PNAS...9212384J. doi:10.1073/pnas.92.26.12384. PMC 40362. PMID 8618906.
- Bonaldo MF, Lennon G, Soares MB (September 1996). "Normalization and subtraction: two approaches to facilitate gene discovery". Genome Research. 6 (9): 791–806. doi:10.1101/gr.6.9.791. PMID 8889548.
- Sourdive DJ, Transy C, Garbay S, Yaniv M (April 1997). "The bifunctional DCOH protein binds to HNF1 independently of its 4-alpha-carbinolamine dehydratase activity". Nucleic Acids Research. 25 (8): 1476–1484. doi:10.1093/nar/25.8.1476. PMC 146627. PMID 9092652.
- Johnen G, Kaufman S (December 1997). "Studies on the enzymatic and transcriptional activity of the dimerization cofactor for hepatocyte nuclear factor 1". Proceedings of the National Academy of Sciences of the United States of America. 94 (25): 13469–13474. Bibcode:1997PNAS...9413469J. doi:10.1073/pnas.94.25.13469. PMC 28329. PMID 9391049.
- Thöny B, Neuheiser F, Kierat L, Blaskovics M, Arn PH, Ferreira P, et al. (June 1998). "Hyperphenylalaninemia with high levels of 7-biopterin is associated with mutations in the PCBD gene encoding the bifunctional protein pterin-4a-carbinolamine dehydratase and transcriptional coactivator (DCoH)". American Journal of Human Genetics. 62 (6): 1302–1311. doi:10.1086/301887. PMC 1377166. PMID 9585615.
- Thöny B, Neuheiser F, Kierat L, Rolland MO, Guibaud P, Schlüter T, et al. (August 1998). "Mutations in the pterin-4alpha-carbinolamine dehydratase (PCBD) gene cause a benign form of hyperphenylalaninemia". Human Genetics. 103 (2): 162–167. doi:10.1007/s004390050800. PMID 9760199. S2CID 7949076.
- Lei XD, Kaufman S (March 1999). "Characterization of expression of the gene for human pterin carbinolamine dehydratase/dimerization cofactor of HNF1". DNA and Cell Biology. 18 (3): 243–252. doi:10.1089/104454999315466. PMID 10098606.
- Waters PJ, Scriver CR, Parniak MA (July 2001). "Homomeric and heteromeric interactions between wild-type and mutant phenylalanine hydroxylase subunits: evaluation of two-hybrid approaches for functional analysis of mutations causing hyperphenylalaninemia". Molecular Genetics and Metabolism. 73 (3): 230–238. doi:10.1006/mgme.2001.3198. PMID 11461190.
- v
- t
- e
-
 1dch: CRYSTAL STRUCTURE OF DCOH, A BIFUNCTIONAL, PROTEIN-BINDING TRANSCRIPTION COACTIVATOR
1dch: CRYSTAL STRUCTURE OF DCOH, A BIFUNCTIONAL, PROTEIN-BINDING TRANSCRIPTION COACTIVATOR -
 1dco: DCOH, A BIFUNCTIONAL PROTEIN-BINDING TRANSCRIPTIONAL COACTIVATOR
1dco: DCOH, A BIFUNCTIONAL PROTEIN-BINDING TRANSCRIPTIONAL COACTIVATOR -
 1dcp: DCOH, A BIFUNCTIONAL PROTEIN-BINDING TRANSCRIPTIONAL COACTIVATOR, COMPLEXED WITH BIOPTERIN
1dcp: DCOH, A BIFUNCTIONAL PROTEIN-BINDING TRANSCRIPTIONAL COACTIVATOR, COMPLEXED WITH BIOPTERIN -
 1f93: CRYSTAL STRUCTURE OF A COMPLEX BETWEEN THE DIMERIZATION DOMAIN OF HNF-1 ALPHA AND THE COACTIVATOR DCOH
1f93: CRYSTAL STRUCTURE OF A COMPLEX BETWEEN THE DIMERIZATION DOMAIN OF HNF-1 ALPHA AND THE COACTIVATOR DCOH
 | This article on a gene on human chromosome 10 is a stub. You can help Wikipedia by expanding it. |
- v
- t
- e