Hispidulin
 | |
| Names | |
|---|---|
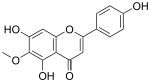
| IUPAC name 4′,5,7-Trihydroxy-6-methoxyflavone | |
| Systematic IUPAC name 5,7-Dihydroxy-2-(4-hydroxyphenyl)-6-methoxy-4H-1-benzopyran-4-one | |
| Identifiers | |
CAS Number |
|
3D model (JSmol) |
|
| ChEBI |
|
| ChemSpider |
|
| ECHA InfoCard | 100.229.713 |
| EC Number |
|
| KEGG |
|
PubChem CID |
|
| UNII |
|
CompTox Dashboard (EPA) |
|
InChI
| |
| |
| Properties | |
Chemical formula | C16H12O6 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). Infobox references | |
Chemical compound
Hispidulin is a naturally occurring flavone with potential antiepileptic activity in rats and gerbils.[1][2] It is found in plants including Grindelia argentina, Arrabidaea chica, Saussurea involucrate, Crossostephium chinense, Artemisia, and Salvia.[3]
Complementary medicine
In traditional and complementary medicine it is claimed to have "antioxidant, antifungal, anti-inflammatory, antimutagenic, and antineoplastic properties".[3]
Notes
- ^ Hispidulin inhibits the release of glutamate in rat cerebrocortical nerve terminals. Lin TY1, Lu CW, Wang CC, Lu JF, Wang SJ. Toxicol Appl Pharmacol. 2012 Sep 1;263(2):233-43. doi: 10.1016/j.taap.2012.06.015. Epub 2012 Jul 1.
- ^ The flavone hispidulin, a benzodiazepine receptor ligand with positive allosteric properties, traverses the blood–brain barrier and exhibits anticonvulsive effects, Dominique Kavvadias et al, British Journal of Pharmacology (2004) 142, 811–820
- ^ a b Kanika Patel, Dinesh Kumar Patel, Medicinal importance, pharmacological activities, and analytical aspects of hispidulin: A concise report, Journal of Traditional and Complementary Medicine, 2016, ISSN 2225-4110, https://dx.doi.org/10.1016/j.jtcme.2016.11.003.
- v
- t
- e
Flavones and their conjugates
| Monohydroxyflavone | |
|---|---|
| Dihydroxyflavones | |
| Trihydroxyflavones | |
| Tetrahydroxyflavones | |
| Pentahydroxyflavones |
|
| O-methylated flavones |
|
| of apigenin | |
|---|---|
| of baicalein | |
| of hypolaetin |
|
| of luteolin |
- Giraldiin A and B
- Nepitrin
- Oroxindin
- Scutellarin
Theograndin I and II
 | This article about an aromatic compound is a stub. You can help Wikipedia by expanding it. |
- v
- t
- e











